If you’ve been tattooed in the US, the ink under your skin may have more components than you previously imagined, a new study reveals. The study, conducted by Binghamton University and titled “What’s in my ink: An analysis of commercial tattoo ink on the U.S. market,” shows discrepancies between the ingredient labels on tattoo ink and the actual substances in the bottle.
When doctoral student Kelli Moseman—the article’s lead author—and researchers Ahs Habibi Ahmed and Alexander Ruhren noted that the tattoo inks they were analyzing contained substances that weren’t on the label, they wondered whether the difference was a result of the ink’s interaction with light.
A new study reveals that the ingredient labels on tattoo ink sold in the US don’t match the actual substances in the bottle
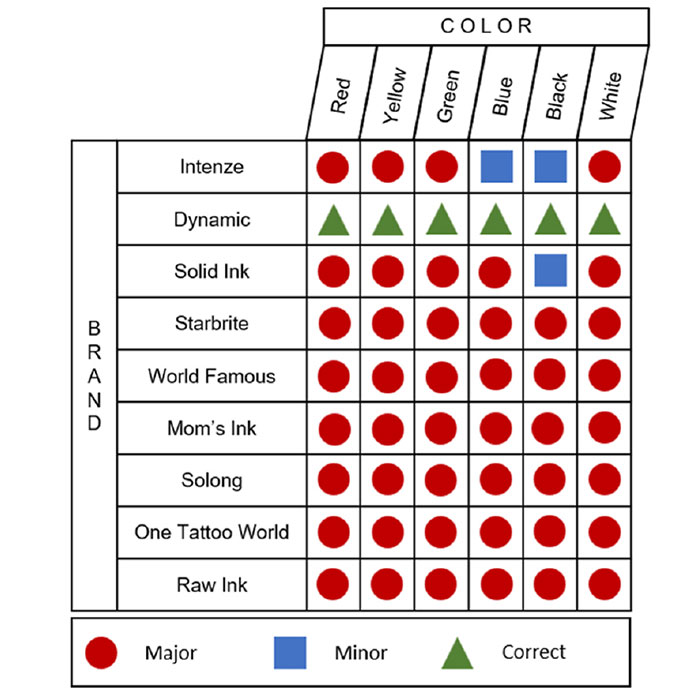
The researchers analyzed tattoo inks from nine manufacturers in the United States, ranging from global companies to smaller producers, and compared their contents with the information listed on the label. The inks in question came in six colors.

After investigating 54 inks, they found that the additional substances were not the result of light exposure, but had been in the product from the start.
What’s more, those wanting to educate themselves on what they were putting under their skin could never have known about this, as the contents were not correctly included in the label.
A surprising 90% of the tested inks contained different pigments than the ones listed or unlisted additives
A surprising 90% of the tested inks (45 of 54) contained different pigments than the ones listed or unlisted additives, researchers discovered.
Most importantly, the unlisted contents could potentially bring health complications to anyone looking to get inked.

While more than half of the inks contained unlisted polyethylene glycol, which can cause organ damage through repeated exposure, 15 contained propylene glycol, a potential allergen.
Other contaminants included an antibiotic commonly used to treat urinary tract infections and 2-phenoxyethanol, which poses potential health risks to nursing infants, as per Phys.org.
“Our goal in a lot of this research is to empower artists and their clients,” Binghamton University Assistant Professor of Chemistry John Swierk said
“We’re hoping the manufacturers take this as an opportunity to reevaluate their processes and that artists and clients take this as an opportunity to push for better labeling and manufacturing,” Binghamton University Assistant Professor of Chemistry John Swierk said.
One of the research’s limitations is that it can’t track the origin of the difference between label and contents. That is, it can’t identify whether the unlisted ingredients were added intentionally or if the manufacturer was provided with incorrectly labeled or contaminated materials.

Two years ago, the American market began being subject to governmental regulation after Congress passed the Modernization of Cosmetics Regulation Act (MoCRA).
This allowed the Food and Drug Administration (FDA) to regulate tattoo inks for the first time, including accurate labeling practices. Previously, inks escaped regulation as they were considered cosmetic in nature.
Swierk hopes the study will help the FDA pay closer attention to the inks under the federal agency’s regulation.
“The FDA is still figuring out what that is going to look like, and we think this study will influence the discussions around MoCRA,” he said.
“This is also the first study to look at inks sold in the United States explicitly and is probably the most comprehensive because it looks at the pigments, which nominally stay in the skin, and the carrier package, which is what the pigment is suspended in.”
The study was conducted by analyzing 54 inks from different colors and brands
As for the European market, the tattoo inks available in the Old Continent are subject to stricter regulations, overseen by the European Chemicals Agency.
Potential risks associated with tattoos focus on skin cancer and the pigments themselves, but additives can also lead to health problems, even beyond the skin.
If someone starts developing issues weeks or years later after getting inked, unlisted ingredients can make it difficult to pinpoint what reaction is happening and why.
Swierk emphasized that the study doesn’t aim to interfere with the job of tattoo artists but to provide clearer information to both workers and clients.
“Our goal in a lot of this research is to empower artists and their clients. Tattoo artists are serious professionals who have dedicated their lives to this craft, and they want the best possible outcomes for their clients,” he added.
“We’re trying to highlight that there are some deficiencies in manufacturing and labeling.”



